A research team at Tokyo University of Agriculture and Technology has developed a solid polymer electrolyte for lithium-ion batteries that balances both ionic conductivity and strength, using carbon dioxide as a raw material. This breakthrough is seen as a step toward the development of next-generation high-efficiency batteries.
A New Approach to Developing Next-Generation Batteries Using Carbon Dioxide
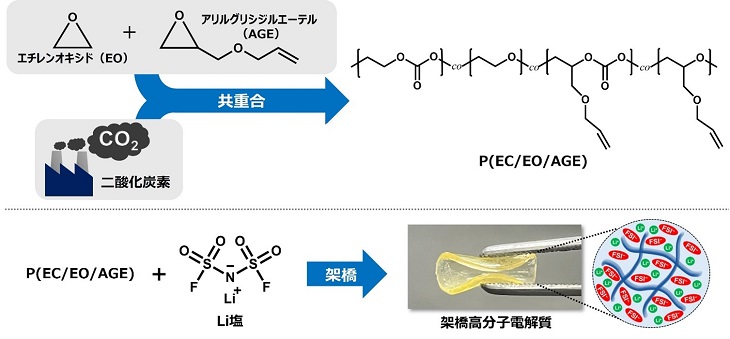
Lithium-ion batteries have become an essential part of modern life, but conventional batteries face challenges in terms of safety and efficiency. Specifically, when using organic electrolytes, there are issues with volatility and flammability, making technological improvements urgent. In response, a research team led by Professor Yoichi Tominaga, Assistant Professor Kento Kimura, and doctoral candidate Nantapat Soontornnon from the Department of Applied Chemistry at the Graduate School of Engineering, Tokyo University of Agriculture and Technology, has developed a solid electrolyte made from carbon dioxide and an epoxide copolymer, achieving a material with unprecedented performance and safety.
Conventional lithium-ion batteries rely on liquid electrolytes using organic solvents, but this research uses a solid polymer derived from carbon dioxide. By copolymerizing carbon dioxide and an epoxide, the team created a new material based on aliphatic polycarbonate, combined with lithium salts. This solid polymer electrolyte offers both high ionic conductivity and mechanical strength, making it applicable as a flexible membrane electrolyte.
The research team precisely controlled the cross-linked structure within the material and increased the concentration of lithium salts, successfully drawing out characteristics not seen in conventional solid polymer electrolytes. This material was tested in secondary batteries using lithium metal as the anode and lithium iron phosphate as the cathode, demonstrating stable performance over more than 400 charge-discharge cycles.
A key highlight of this research is the balance achieved between high ionic conductivity and strength in the polymer material made from carbon dioxide. Previous materials faced a dilemma where enhancing the flexibility or durability of the electrolyte compromised its ionic conductivity. However, the team led by Professor Tominaga solved this issue by introducing a cross-linked structure within the material.
While cross-linking has generally been used to improve the strength of materials, it often led to a decrease in ionic conductivity. In this study, the unique properties of the new copolymer containing carbon dioxide allowed the introduction of cross-linking without sacrificing ionic conductivity. This innovative design maintained the function of the electrolyte as a separator while enabling efficient charge-discharge cycles.
This new material not only improves the safety and performance of lithium-ion batteries but also has significant environmental implications through the effective use of carbon dioxide. As the world moves towards carbon neutrality, this achievement is gaining attention as a new method for producing high-performance materials while sequestering carbon dioxide.
Building on these results, the research team aims to further evolve material design, with future plans to develop lithium-ion batteries that operate at higher voltages and to explore applications of next-generation batteries using alternative elements such as sodium.
Lithium-ion batteries are currently used in a wide range of applications, from smartphones to electric vehicles, and their continued evolution is key to future technological advancements. This research represents an important step toward realizing safer, higher-performance batteries. In particular, as efficient and environmentally friendly energy storage technologies are sought to support the spread of renewable energy and electric vehicles, the impact of these research results is expected to be significant.
Visit the press release here
