A research team from Kyoto Sangyo University and Kyoto Institute of Technology has uncovered the rotational mechanism of ATP synthase, a key enzyme in cellular energy production. Using cryo-electron microscopy and molecular dynamics simulations, they elucidated how proton flow drives the enzyme’s rotation. These findings will be published in Nature Communications on November 20, 2024.
Capturing the Mechanism of ATP Production: Implications for Drug Discovery
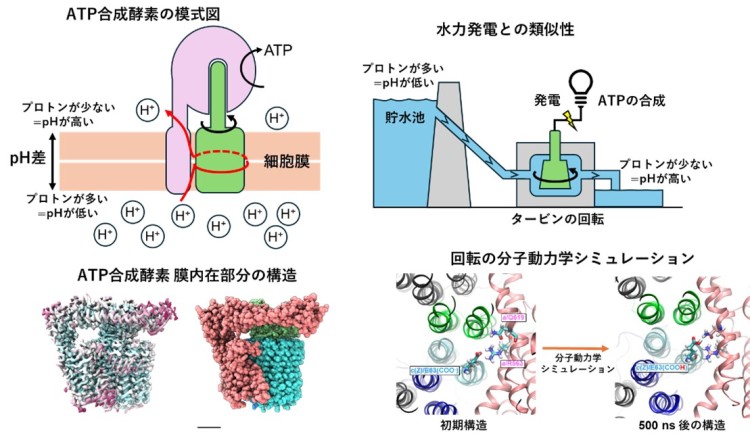
ATP (adenosine triphosphate), the molecule that powers life, is synthesized by ATP synthase, a massive protein complex that converts the energy of proton flow across mitochondrial membranes into rotational motion. This rotation fuels ATP production, but its molecular mechanism has remained elusive for decades.
In this study, the research team analyzed the membrane-embedded portion of ATP synthase derived from the thermophilic bacterium Thermus thermophilus. Using cryo-electron microscopy, they resolved its atomic-level structure, and through molecular dynamics simulations, they revealed that specific amino acid residues in the rotary ring change their conformation depending on proton-binding states. This conformational change creates an asymmetry that drives unidirectional rotation.
According to the researchers, these insights not only advance fundamental understanding of ATP synthase but also open avenues for developing treatments for diseases like diabetes and mitochondrial disorders. Moreover, the methodologies employed in this research are adaptable to other membrane proteins, suggesting broader applications.
Moving forward, the team plans to extend their investigations to ATP synthase in human mitochondria, aiming to make further breakthroughs in both life sciences and drug discovery.
